pH scale
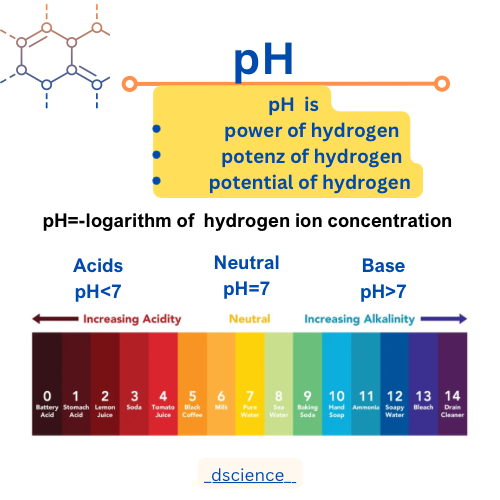
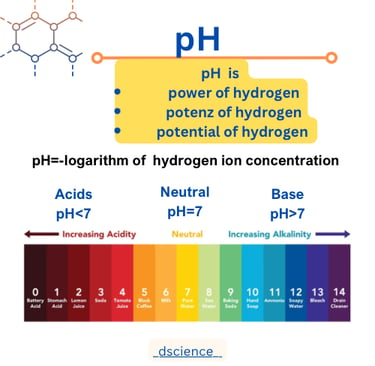
pH
pH, which stands for "power of hydrogen," is a measure of the acidity or alkalinity of a solution. It quantifies the concentration of hydrogen ions (H+) present in a substance. The pH scale ranges from 0 to 14, with 7 being neutral. A pH below 7 indicates acidity, while a pH above 7 indicates alkalinity.
History of pH
The concept of pH was first introduced by Danish chemist Søren Peder Lauritz Sørensen in 1909. Sørensen developed the pH scale as a way to measure the acidity of solutions in the Carlsberg Laboratory, where he worked. His groundbreaking work revolutionized the field of chemistry and provided scientists with a standardized method to measure acidity.
pH Scale
The pH scale is a logarithmic scale, meaning that each unit represents a tenfold difference in acidity or alkalinity. For example, a substance with a pH of 3 is ten times more acidic than a substance with a pH of 4. The pH scale is often depicted as a vertical line with numbers ranging from 0 to 14, where 7 is the midpoint.
Substances with a pH below 7 are considered acidic. The lower the pH value, the more acidic the substance. Examples of acidic substances include lemon juice (pH 2), vinegar (pH 3), and stomach acid (pH 1-2).
On the other hand, substances with a pH above 7 are considered alkaline or basic. The higher the pH value, the more alkaline the substance. Examples of alkaline substances include baking soda (pH 9), soap (pH 10), and bleach (pH 12).
Formula of pH
The formula for calculating pH is:
pH = -log[H+]
Here, [H+] represents the concentration of hydrogen ions in moles per liter (mol/L) or molarity. The negative logarithm of the hydrogen ion concentration gives the pH value of the solution.
It is important to note that pH is a dimensionless quantity and does not have units. It is solely a measure of the concentration of hydrogen ions in a solution.
Importance of pH
pH plays a crucial role in various aspects of life and science. It affects the properties and behavior of substances, chemical reactions, and biological processes. Understanding pH is essential in fields such as chemistry, biology, environmental science, and medicine.
In the human body, pH balance is critical for maintaining optimal health. The pH of blood, for example, must remain within a narrow range of 7.35 to 7.45 for proper functioning of bodily systems. Imbalances in pH can lead to health issues and complications.
Additionally, pH is important in industries such as agriculture, water treatment, and food production. It helps determine the suitability of soil for plant growth, the quality of drinking water, and the preservation of food products.
In conclusion, pH is a fundamental concept in the world of science and has far-reaching implications in various fields. Understanding pH and its measurement on the pH scale is crucial for comprehending the properties of substances and their impact on our daily lives.