Indicators
Introduction to Indicators
An indicator is a substance that undergoes a distinct color change when it is exposed to certain conditions, such as changes in pH or the presence of specific chemicals. Indicators are widely used in various fields, including chemistry, biology, and medicine, to determine the presence or absence of certain substances or to measure the acidity or alkalinity of a solution. In this article, we will focus on one specific indicator called phenolphthalein and explore its properties and the color change it undergoes.
Types of Indicators
Indicators can be classified into different types based on their chemical properties and the type of color change they undergo. Some indicators are natural substances, while others are synthetic compounds. Common types of indicators include litmus paper, pH paper, bromothymol blue, and phenolphthalein.
Phenolphthalein
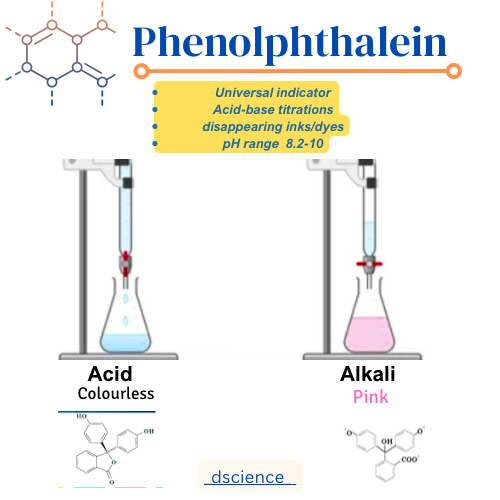
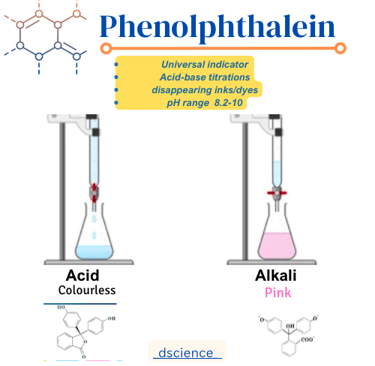
Phenolphthalein is a synthetic indicator that is widely used in acid-base titrations and other analytical procedures. It is a colorless compound in its acidic form and turns pink or magenta when it is exposed to a basic solution. Phenolphthalein is often used because of its sharp and distinct color change, making it easy to detect the endpoint of a titration.
Color Change in Phenolphthalein
The color change in phenolphthalein occurs due to changes in the pH of the solution. In acidic solutions, phenolphthalein remains colorless because the hydrogen ions present in the solution prevent the formation of the pink color. However, when the solution becomes basic, the hydroxide ions react with the phenolphthalein, causing the compound to turn pink or magenta.
pH Range
The pH range at which phenolphthalein undergoes the color change is around pH 8.2 to pH 10.0. Below pH 8.2, the solution remains colorless, and above pH 10.0, the solution remains pink or magenta. This pH range makes phenolphthalein suitable for detecting the endpoint of acid-base titrations, as it provides a clear indication of when the solution has reached neutrality or the desired pH.
Structure of Phenolphthalein
The structure of phenolphthalein consists of a complex aromatic ring system with two phenol groups attached to it. The presence of these phenol groups is responsible for the color change in phenolphthalein. The exact mechanism of the color change is still a subject of scientific research, but it is believed to involve the formation of a conjugated system and the interaction of the phenolphthalein molecule with the solvent.
Conclusion
Indicators, such as phenolphthalein, play a crucial role in various scientific and analytical processes. Understanding the properties and behavior of indicators, including the color change in phenolphthalein, allows scientists and researchers to accurately measure and analyze the acidity or alkalinity of a solution. Phenolphthalein's distinct color change and its pH range make it a valuable tool in acid-base titrations and other chemical analyses.