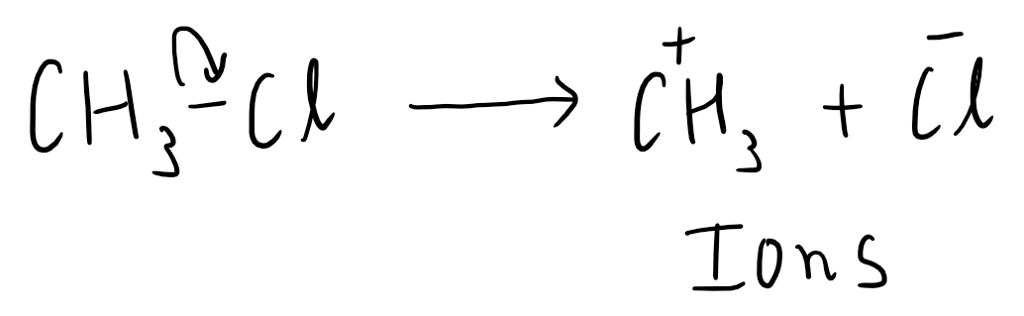Homolytic and Heterolytic fission
Types of Fission
Fission refers to the breaking of a covalent bond, resulting in the separation of the bonded atoms. There are two primary types of fission: homolytic fission and heterolytic fission.
1. Homolytic Fission
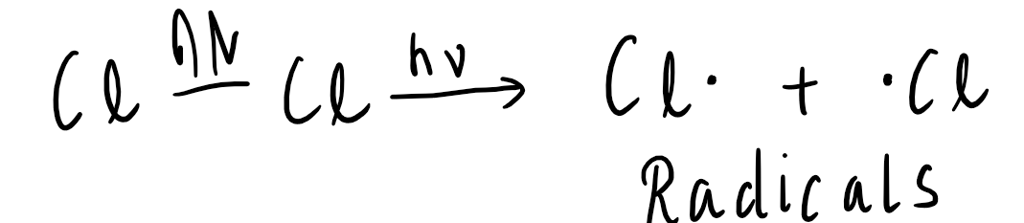
Homolytic fission occurs when a covalent bond breaks, and each atom retains one of the shared electrons. This results in the formation of two highly reactive species called radicals. Radicals are atoms or molecules that have unpaired electrons, making them highly reactive and prone to forming new bonds.
Homolytic fission is typically initiated by the input of energy, such as heat or light. This process is common in organic chemistry reactions and plays a crucial role in the synthesis and degradation of organic compounds.
2. Heterolytic Fission
Heterolytic fission occurs when a covalent bond breaks unevenly, resulting in one atom retaining both of the shared electrons. This leads to the formation of two charged species called ions. The atom that gains the electrons becomes negatively charged (anion), while the atom that loses the electrons becomes positively charged (cation).
Heterolytic fission usually occurs in polar covalent bonds, where there is a significant difference in electronegativity between the atoms involved. This type of fission is commonly observed in ionic compounds and certain organic reactions.
What is a Covalent Bond?
A covalent bond is a type of chemical bond that occurs when two atoms share electrons. It is formed between non-metal atoms that have similar electronegativities, meaning they have a similar tendency to attract electrons. In a covalent bond, the shared electrons orbit around the nuclei of both atoms, creating a stable molecular structure.
Covalent bonds are essential for the formation of molecules and are responsible for the stability of many compounds, such as water (H2O), methane (CH4), and carbon dioxide (CO2). These bonds are characterized by their strength and the energy required to break them.
The fission of a covalent bond is a fundamental process in chemistry, playing a crucial role in the formation and transformation of molecules. Understanding the different types of fission, homolytic and heterolytic, allows scientists to predict and control chemical reactions, leading to advancements in various fields, including pharmaceuticals, materials science, and environmental studies.
By studying the fission of covalent bonds, researchers can gain insights into the behavior of atoms and molecules, enabling them to develop new compounds and understand the intricacies of chemical reactions.