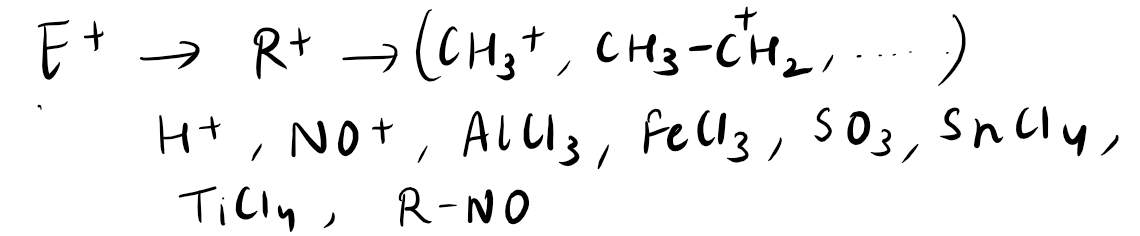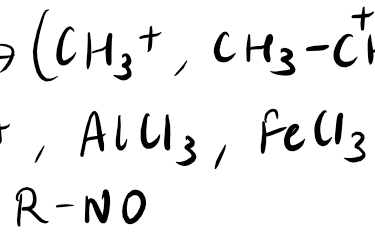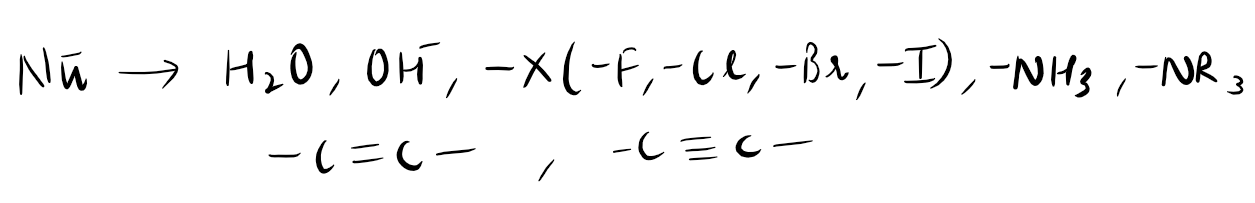Electrophiles and Nucleophiles
In organic chemistry, understanding the concepts of electrophiles and nucleophiles is crucial. These terms refer to molecules or ions that participate in chemical reactions by either accepting or donating electron pairs.
Electrophiles are electron-deficient species that seek electron-rich sites, while nucleophiles are electron-rich species that seek electron-deficient sites. Let's delve deeper into the types and examples of electrophiles and nucleophiles.
Electrophiles
Electrophiles are chemical species that are electron-deficient and tend to react with electron-rich species, known as nucleophiles. These reactive molecules are attracted to regions of high electron density and seek to gain electrons to achieve a more stable state. Electrophilic reactions are commonly involved in organic chemistry, where electrophiles react with nucleophiles to form new chemical bonds. One of the most well-known examples of an electrophilic reaction is the addition of an electrophile, such as a hydrogen ion, to an alkene to form an alkane. Electrophiles play a crucial role in various chemical reactions and are essential for the synthesis of many organic compounds.
Electrophiles can be categorized into various types based on their electron-deficient nature and their mode of reactivity. Some common types of electrophiles include:
Acids: Acidic compounds, such as carboxylic acids or mineral acids, can act as electrophiles by accepting electron pairs.
Carbocations: Carbocations are positively charged carbon atoms that are electron-deficient and highly reactive.
Electron-deficient atoms: Atoms like boron, aluminum, and sulfur, which have incomplete octets, can act as electrophiles.
Electrophilic reagents: These are specific chemicals designed to react as electrophiles, such as acyl halides or alkyl halides.
Nucleophiles
Nucleophiles are chemical species that have a strong affinity for positively charged atoms or regions in molecules. They play a crucial role in many chemical reactions, particularly in organic chemistry. Nucleophiles are electron-rich and can donate a pair of electrons to form a new bond with an electron-deficient atom or group. This process is known as nucleophilic attack. Nucleophiles can be negatively charged, such as hydroxide ions (OH-) or cyanide ions (CN-), or have lone pairs of electrons, like amines. They are often involved in substitution reactions, where one atom or group is replaced by another. Nucleophiles are essential in various biological processes and are widely used in synthetic chemistry to create new compounds.
Nucleophiles can also be classified into different types based on their electron-rich nature and their mode of reactivity. Some common types of nucleophiles include:
Alkoxides: Alkoxides are negatively charged species derived from alcohols and are excellent nucleophiles.
Grignard reagents: These are organometallic compounds containing a carbon-magnesium bond and are highly reactive nucleophiles.
Amines: Amines, which contain a lone pair of electrons on the nitrogen atom, can act as nucleophiles.
Hydroxide ions: OH- ions are strong nucleophiles due to their high electron density.
Understanding electrophiles and nucleophiles is essential for comprehending the mechanisms of organic reactions. Electrophiles seek electron-rich sites, while nucleophiles seek electron-deficient sites. By recognizing the different types and examples of electrophiles and nucleophiles, chemists can predict and manipulate chemical reactions more effectively.