Bredt's Rule: Chemistry Evolves, Textbooks to Follow
8/23/20254 min read
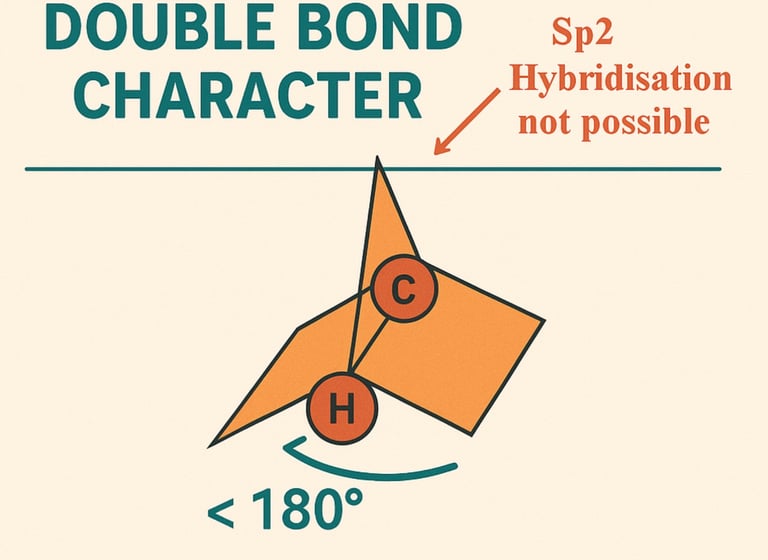
Bredt’s rule:100-year-old chemistry rule proven false, textbook recalibration needed
Understanding Bredt’s Rule
Bredt’s Rule, introduced by the chemist E.J. Bredt in 1921, has played a pivotal role in the development and understanding of organic chemistry. This principle pertains to the stability of cyclic compounds, emphasizing that certain bridged bicyclic systems cannot exist if they contain a double bond at a bridgehead position. Essentially, Bredt's Rule asserts that while cycloalkenes can theoretically adopt configurations that allow for double bonds, the presence of a double bond at specific locations often renders the molecule unstable. This insight has had a profound impact on the study of molecular structures and reactions.
Over the last century, Bredt’s Rule has shaped the way chemists approach complex organic reactions and the synthesis of various compounds. The rule facilitates predictions regarding molecular behavior, guiding researchers in their efforts to design and synthesize new molecules. The implications of Bredt’s Rule extend beyond merely outlining the stability of cyclic compounds; it serves as a critical parameter in assessing reactivity and potential pathways in synthesis. Consequently, this principle not only informs theoretical frameworks but also assists in practical applications within organic chemistry.
Furthermore, the advancements in computational chemistry and molecular modeling have enabled an even deeper exploration of Bredt’s Rule. Researchers can now simulate various cyclic systems to analyze their stability based on this rule, leading to valuable insights into the behavior of unstable compounds. As new discoveries continue to emerge, the relevance of Bredt’s Rule remains firmly entrenched. Overall, the rule stands as a testament to the evolving nature of chemical understanding, proving that foundational concepts can have lasting impacts on scientific inquiry and innovation in organic chemistry.
Breaking Bredt’s Rule
In recent years, the understanding of Bredt's Rule has undergone significant scrutiny, particularly with the findings from a research team at UCLA. Traditionally, Bredt's Rule has stipulated that the formation of certain bridgehead double bonds in bicyclic compounds is prohibited due to angle strain. The UCLA team aimed to test this long-accepted chemical principle using a novel experimental design that involved monitoring the breakdown of specific cyclic compounds without the need for prior isolation.
The researchers utilized a combination of advanced spectroscopic techniques, which included nuclear magnetic resonance (NMR) and mass spectrometry, to observe chemical reactions in real-time. This innovative approach enabled them to capture the dynamics of molecular interactions and transformations as they occurred, rather than relying solely on static, isolated samples. The study focused on various bicyclic compounds known to violate Bredt's Rule during theoretical predictions, thus placing the rule under experimental challenge.
Throughout the study, the team carefully analyzed the potential energy surfaces of the compounds, observing how bond formation and breaking occurred under specific conditions. Through these observations, they uncovered instances where bridgehead double bonds did, in fact, form despite the traditional perspective dictated by Bredt’s Rule. The results indicated that molecular factors, such as steric hindrance and electronic effects, played a more complex role in determining a compound's stability than previously thought.
The outcomes of this research not only question the rigid interpretation of Bredt's Rule but also emphasize the importance of experimental validation in the field of organic chemistry. The UCLA team's findings suggest that future studies may explore more exceptions to the rule, which could lead to the development of novel methodologies in organic synthesis. This paradigm shift underscores the evolving nature of chemical principles and the necessity to continually reassess established theories in light of new evidence.
Beyond Bredt: A Modern Take on a Classic Rule
Bredt's Rule, formulated over a century ago, has long served as a foundational guideline in organic chemistry, dictating the stability of certain bicyclic compounds. Recent discoveries, however, have prompted a critical reevaluation of this principle. Chemists are now faced with compelling evidence suggesting that molecular stability and reactivity may not align as strictly with Bredt's Rule as previously believed. This paradigm shift necessitates an updated understanding and may lead to significant revisions in established practices within the field.
As researchers delve deeper into the complexities of molecular structures, it becomes increasingly apparent that Bredt's Rule might not uniformly apply across all scenarios. The implications of these findings suggest that textbooks and educational resources—often regarded as the cornerstone of chemical education—require a thorough reexamination. By incorporating these novel insights into educational curricula, educators can better prepare the next generation of chemists to navigate the evolving landscape of organic chemistry.
Moreover, embracing these revelations serves not only to revise existing knowledge but also to stimulate new avenues for future research. Understanding the conditions under which Bredt's Rule can be challenged opens doors to the exploration of previously overlooked compounds. This could lead to the discovery of new materials and catalysts, ultimately advancing both theoretical and applied chemistry.
Philosophically, these developments highlight the nature of scientific progress. As the community revisits established principles like Bredt's Rule, it fosters an environment where creativity and critical thinking are essential. This spirit of inquiry not only enriches the discourse within the scientific community but also exemplifies the dynamic nature of knowledge in the realm of organic chemistry.
What Makes This Relevant?
The recent overturning of Bredt's Rule holds significant implications across multiple domains, including educational institutions, research opportunities, and industrial applications in organic chemistry. As one of the foundational principles in organic chemistry, the refinement of this century-old rule challenges long-maintained beliefs and encourages a reassessment of methodologies in both academic and industrial settings. Educational institutions may be prompted to revise their curricula, integrating this new understanding into their teaching modules. This evolution can enhance students' grasp of chemical principles and encourage innovative thinking among future scientists.
Moreover, this paradigm shift opens new avenues for research. Scientists may engage in further inquiries, exploring previously dismissed pathways in organic synthesis and molecular designs. The study’s publication in a highly regarded journal such as Science not only elevates its credibility but also serves as an impetus for researchers to delve deeper into the nuances of molecular behavior. Such advancements could lead to a plethora of novel compounds, fostering innovation within the pharmaceutical and materials industries.
Industrial applications stand to benefit tremendously from the updated interpretation of Bredt's Rule. As chemists explore previously overlooked structures, industries such as pharmaceuticals can develop more effective drugs, while materials science might yield stronger and more versatile materials. This ongoing evolution in our understanding of organic chemistry is a call to action; it emphasizes the necessity for the scientific community to remain receptive to change and questioning established norms. By doing so, researchers across various fields can facilitate meaningful advancements, ultimately enriching scientific knowledge and practical applications. The implications of this breakthrough extend far beyond chemistry, suggesting the importance of critical evaluation across all scientific disciplines.
This article builds on insights originally reported by Earth.com, whose coverage provided valuable background on the topic.
Contacts
Socials
Subscribe to our newsletter
support@otgscience.in
Copyright © 2024 otgscience.in
.
